Is Bio-Electric Medicine the Next Frontier of Therapy for Coronavirus Victims?
Just Published – Dr. Patrick Nemechek proposes new technology to suppress COVID-19 cytokine storm.
Overland Park, Kansas, June 29, 2020 – Nemechek Technologies, LLC announced the publication of a peer-reviewed article by Patrick Nemechek D.O. in The Journal of Integrative Clinical Medicine. Entitled, ‘Transcutaneous Auricular Vagus Nerve Stimulation Holds the Potential to Suppress the COVID-19 Cytokine Storm’, the article explains the science behind the inflammatory reflex and how vagus nerve stimulation may be a cost-effective and life-saving therapy.
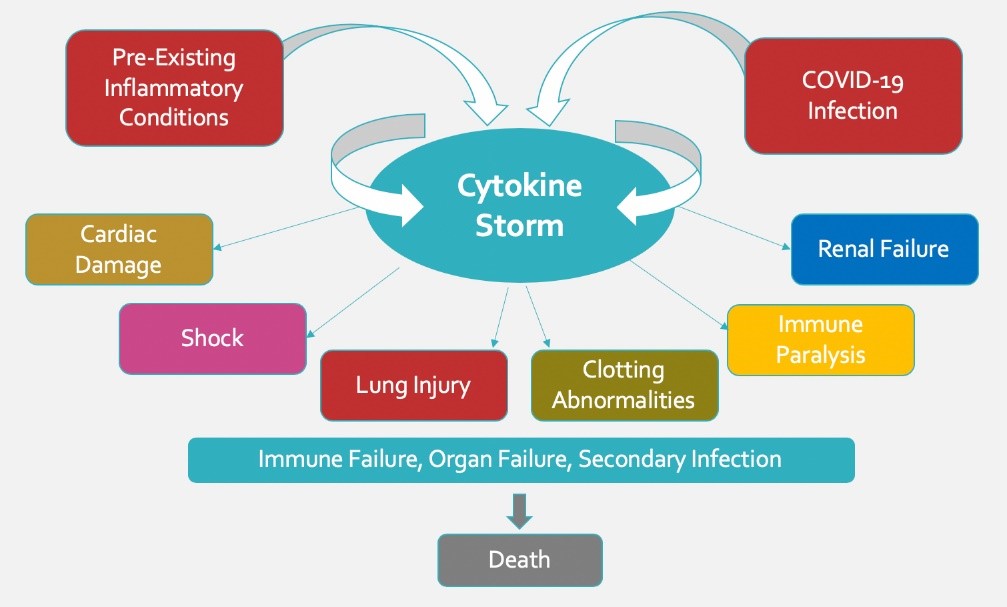
Patients hospitalized with COVID-19 are predominantly dying of respiratory failure due to a surge of pro-inflammatory protein molecules called cytokines that regulate inflammation. In some COVID-19 patients, an excessive inflammatory reaction known as a “cytokine storm” will trigger acute respiratory distress syndrome. This severe form of lung injury is why many patients require mechanical ventilation.
Dr. Nemechek argues that a potential solution to this deadly, unregulated immune response may be a mild electrical current applied to the vagus nerve. His method, known as transcutaneous auricular vagus nerve stimulation (taVNS), delivers a barely perceptible micro-current through a specially designed clip that fits comfortably on the ear.
Stimulation of the afferent vagus nerve fibers in certain portions of the ear activates the inflammatory reflex. Much like the baroreflex that controls blood pressure, the inflammatory reflex signals the body to naturally regulate an immune response. Human studies show that VNS is effective in suppressing a range of pro-inflammatory cytokines in septic shock and improves clinical symptoms in a range of pathologies. Additionally, animal studies suggest that VNS may also be capable of mitigating abnormal clotting seen in more severe COVID-19 patients.
taVNS is largely unrecognized as a therapeutic tool to regulate the cytokine storm, and potentially coagulation abnormalities, in hospitalized patients. It is safe, non-invasive, easy to us, and does not interfere with other therapies. Importantly, a single taVNS device can be shared among many patients with minimum sterilization.
“A clinical trial is urgently needed. Resource-limited healthcare systems world-wide need a cost-effective treatment to reduce COVID-19 ICU admissions, patient intubations, and deaths”.
– Patrick Nemechek D.O.